

RMP lists already confirmed ADRs (important identified risks). Sharing the published information among medical professionals is meant to ensure further enhancements of post-marketing safety measures. The RMP aims that the risks of drugs are evaluated at regular intervals or in response to the progress of post-marketing surveillance and a set of pharmacovigilance activities to minimize the risks of drugs. The RMP is a document which shows the consistent risk management of drug from the development phase to the post-marketing phase. Over his nearly 30-year career, he worked with numerous companies as an employee and a consultant to build or improve complaint analysis, trending, post-market surveillance (PMS), nonconformance (NC), corrective action/preventive action (CAPA), stewardship, and risk management processes.In order to ensure the safety of drugs, it is important to assess measures for appropriate management of the risks of drugs at any time from the development phase to the regulatory review and the post-marketing phase. Joe Simon holds an MBA and has been a member of ASQ since 2008. Creating post-market surveillance (PMS) processes is essential to determine if additional clinical/performance studies are necessary.Developing labels and instructions can help end-users and patients clearly understand the pertinent risks.Creating useable and audit-ready risk documents can support verification/validation (V/V) sampling plans.Using consistent language in a holistic, closed-loop risk management system leads to greater efficiency.Designing product and manufacturing processes controls risks.This book can help you build a “bulletproof” risk process.
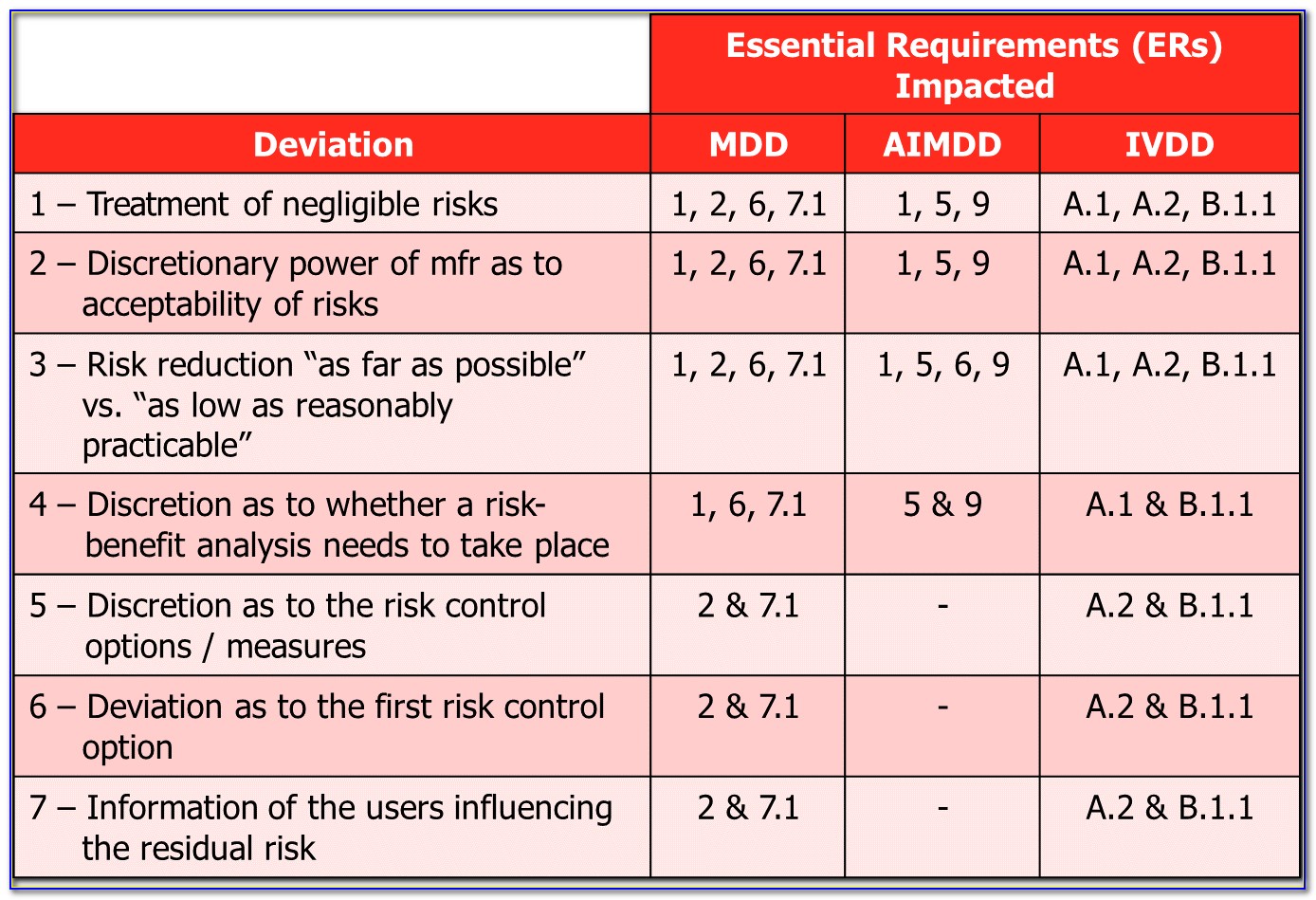
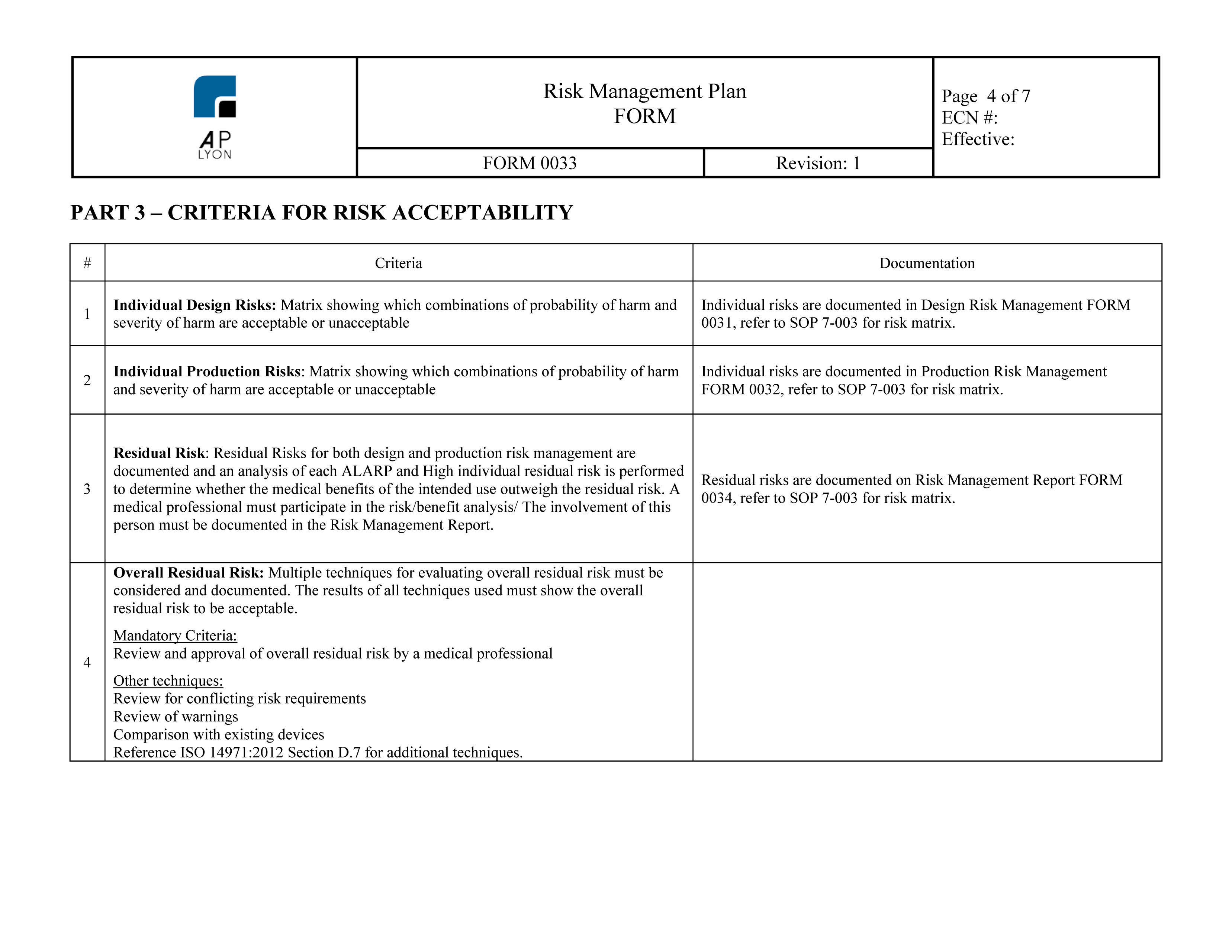
Industry regulations and standards-like ISO 14971-help medical device manufacturers define risk management processes, but they don’t make them “bulletproof”-that is, ensure the efficacy of their products while minimizing future liability.
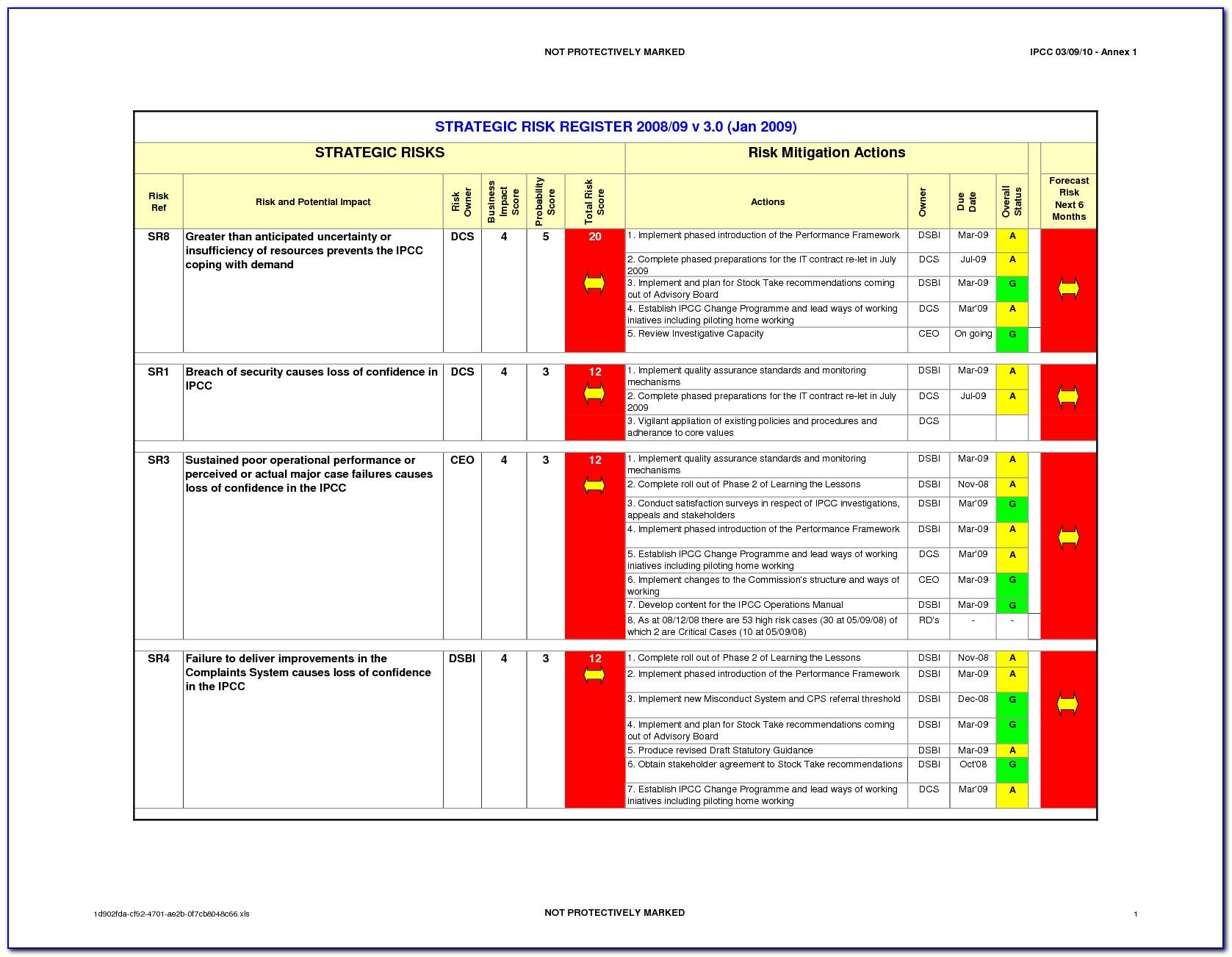
As a quality professional in the medical device industry, you know all too well the importance of a risk management process-and how iterative it can be.


 0 kommentar(er)
0 kommentar(er)
